Methanobrevibacter - What we know about the most abundant archaea in the human gut
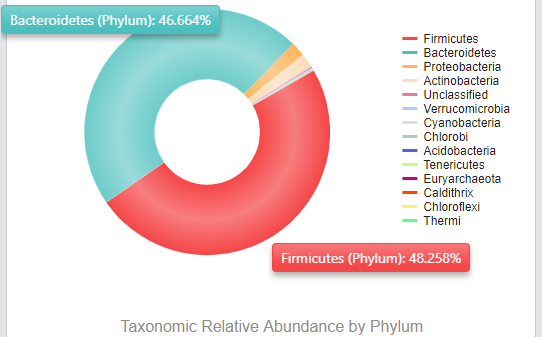
Here at Biomesight, our primary focus is on gut bacteria as these comprise most of the microbiome. Most notably are the phyla Bacteroides and Firmicutes, which make up almost all microorganisms in the microbiome. This can be seen in my sample below.
However, the gut is not only colonised by bacteria but also the other domains of life, Archaea and Eukarya, both of which play no small part in gut health. The gut is much more prevalent with Archaea than Eukarya, with the majority of the Archaea found in the gut belonging to the genus Methanobrevibacter. The amount of Archaea found in the gut widely varies between people, with studies finding ranges between 0.1 to 10.24 ± 4.58% coverage of the microbiome [1].
But what are Methanobrevibacter?
This genus of Archaea are methanogens, meaning that the microbes belonging to this genus produce methane. The most prevalent species from this genus is M. smithii, overshadowing the other most prevalent species, M. stadtmanae. This is because M. smithii is more well suited to the environment of the gut microbiome. M. smithii converts the readily available hydrogen gas, carbon dioxide, and formate into methane. This contrasts with M. stadtmanae, which is limited to using hydrogen gas, acetate, and methanol to make methane. These methanogens need to do this since these reactions sustain the production of energy for themselves via anaerobic respiration [2,3]. As M. smithii has a much greater presence in the microbiome, this will be the main focus of this article.
M. smithii is in much higher numbers than M. stadtmanae because bacterial fermentation causes the production of SCFAs, other organic acids, alcohols, and gases such as hydrogen. Methanogens prevent the buildup of these gases and other reaction end products so the other microorganisms can continue with fermentation unimpeded. This is important because host absorption of SCFAs provide up to 10% of daily caloric intake, and the accumulation of gases such as hydrogen would decrease the efficiency of microbial fermentation and, thus, the yield of nutrients [4].
What does Methanobrevibacter do?
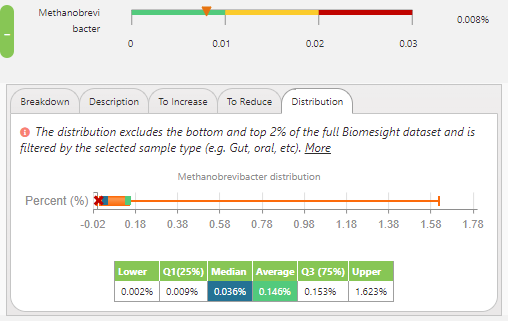
So, looking at the dataset from the samples in Biomesight, the median and average relative abundance for Methanobrevibacter are 0.036% and 0.146%, respectively. This seems to imply that many of us are varying from the optimum range, which has been established as a relative abundance between 0 to 0.01% Methanobrevibacter, derived from the teachings of Dr Jason Hawrelak from Probiotic Advisor.
But what does having different amounts of Methanobrevibacter do? To date, there have been many studies on Methanobrevibacter, but despite this breadth of knowledge, clear conclusions have yet to be found. While many of the studies published imply that having lower quantities is beneficial, other studies claim the opposite.
While the mechanisms are unclear, it has been reported that having higher quantities of M. smithii is detrimental, so logically, lower quantities would be beneficial since it helps combat the diseases associated with M. smithii:
- The relative abundance of Methanobrevibacter in the gut is highly correlated with the degree of flatulence [5]
- Paradoxically, less Methanobrevibacter results in more hydrogen gas being present, which also contributes to flatulence
- This increased flatulence caused by hydrogen is similarly seen in lactose intolerance and small intestinal bacterial overgrowth [6,7]
- This increased flatulence caused by hydrogen is similarly seen in lactose intolerance and small intestinal bacterial overgrowth [6,7]
- Paradoxically, less Methanobrevibacter results in more hydrogen gas being present, which also contributes to flatulence
- Positive correlation between breath methane and M. smithii in C-IBS patients [8]
- Methanobrevibacter presence in the gut was found to be increased in patients with multiple sclerosis [9]
- More specifically, as the number of M. smithii increased, inflammatory responses increased, causing damage associated with multiple sclerosis
- While not directly tied to the gut, it can also affect other parts of the body
- M. smithii found in muscle abscesses [10]
- M. smithii found in the lungs of patients with severe pneumonia [11]
- M. smithii found together with M. oralis in the sinuses of patients with refractory sinusitis [12]
- Although UTIs are typically associated with bacteria, M. smithii has been found co-cultured with bacteria in urine samples from patients with UTIs [13]
- M. smithii was reported to be found in the vagina only in patients suffering from vaginosis and was absent in healthy individuals [14, 15]
- It has been suggested that M. smithii is involved in mixed infections by promoting the growth of the pathogenic bacteria through mutually beneficial interactions [16]
- While unclear exactly how, it is thought that this is because methanogens control the production of essential substances used by these bacteria in diseases such as tonsillar phlegmon
- M. smithii found in muscle abscesses [10]
Other studies claimed that having higher levels of M. smithii is beneficial
- M. smithii presence in stool samples of IBD (the main two being Crohn’s disease and ulcerative colitis) patients was significantly lower than healthy control subjects [17]
- To further support this, it was found that in remission, more M. smithii was present
- The presence of methanogens in renal transplant recipients is significantly lower when compared to healthy controls, which is similar to what is seen in IBD patients [18]
- It can also be beneficial in other diseases
- M. smithii, alongside two other methanogenic archaea, showed a protective effect on a mouse model prone to atherosclerosis [19]
Where it becomes complicated is because many studies have conflicting results with M. smithii and weight.
- Some studies reported that M. smithii, alongside B. animalis was associated with an average weight [20]
- Some studies have found that the gut microbiota associated with human obesity has a positive correlation with M. smithii
- In mice, it was speculated that M. smithii was found to enhance gut microbiota to digest dietary polysaccharides, leading to obesity via increased fat deposition [21]
- Children between 6 and 10 more colonised with M. smithii gained weight more than those less colonised [22]
- In mice, it was speculated that M. smithii was found to enhance gut microbiota to digest dietary polysaccharides, leading to obesity via increased fat deposition [21]
- Some studies have found that higher levels of M. smithii is associated with a lean phenotype in humans
- Studies found that depletion of M. smithii was linked to obesity, M. smithii being negatively correlated with fat [23]
- Christensenella, a genus of bacteria, positively associates with M. smithii since it provides M. smithii with hydrogen needed for methanogenesis [24]
- Christensenella has been found to correlate with leanness, so, logically, M. smithii would have a higher prevalence in lean individuals expressing higher levels of Christensenella
- Studies found that depletion of M. smithii was linked to obesity, M. smithii being negatively correlated with fat [23]
- What seems to be a commonly found result is that M. smithii presence is highly correlated with anorexic patients, with the number of M. smithii being significantly higher in anorexic patients compared to obese or lean individuals
- M. smithii was highly significantly associated with the absence of severe acute malnutrition and therefore can be used as a probiotic to restore gut symbiosis [25]
- Higher abundance of M. smithii is thought to be an adaptive attempt to optimise food transformation in low caloric diets [26]
- Higher abundance of M. smithii explains the clinical symptoms of constipation found in anorexic patients since M. smithii would increase gut methane levels, directly inhibiting gastrointestinal motility [27]
- It was found that using an antibiotic reduced M. smithii levels and relieved these symptoms
It should also be noted that some studies found methanogens are positively correlated with age [24].
So what we can conclude from this is that the literature for Methanobrevibacter is highly disputed, especially on the topic of weight gain or loss. It seems we cannot put a blanket statement on whether having Methanobrevibacter in higher or lower levels is beneficial or not, but rather is something specific to the individual. For example, it was reported that having higher levels of Methanobrevibacter is desired in individuals with IBD, but lower levels are desired when an individual has multiple sclerosis.
Using our new cohort filter feature allows us to see if these changes in Methanobrevibacter can be reflected in our dataset. I can compare myself to people with IBD by selecting to filter results to only display those with Crohn’s disease or ulcerative colitis.
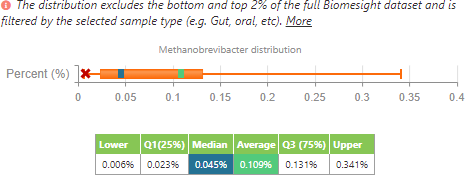
M. smithii distribution filtering to people with Crohn’s disease or ulcerative colitis.
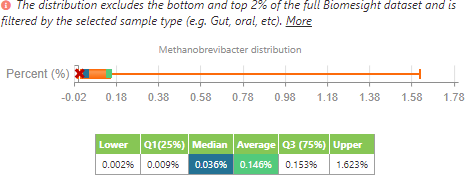
M. smithii distribution of all individuals in the dataset.
From this, we can see that, in accordance with the literature, the average of M. smithii presence is lower in individuals with IBD when compared to all the other individuals in the dataset. However, as to be expected, the range is smaller since it has been filtered to a lower number of individuals (24 samples for IBD compared to 1840 samples for the control group). In contrast to the literature, the median of M. smithii in this small sample set is currently higher in those with IBD than those in the control group.
What should we conclude?
With conflicting research findings and still a relatively small sample set, we are not yet in a position to adjust our ranges from Dr Hawrelak's ideal range recommendations. As many of us have learned, gut microbiome health is about the overall ecosystem more than the individual relative abundances of specific microbes. This seems to be particularly true in the case of M. smithii. If your Methanobrevibactor is out of range, our suggestion is to consider whether your symptoms correspond to those associated with higher numbers and to only focus on reducing it in that case. Recommendations for reducing Methanobrevibactor include taking allicin or PHGG or consuming garlic.
References
1. Kim J, Whon T, Lim M et al. The human gut archaeome: identification of diverse haloarchaea in Korean subjects. Microbiome. 2020;8(1). doi:10.1186/s40168-020-00894-x
2. Deppenmeier U. The unique biochemistry of methanogenesis. Prog Nucleic Acid Res Mol Biol. 2002:223-283. doi:10.1016/s0079-6603(02)71045-3
3. Fricke W, Seedorf H, Henne A et al. The Genome Sequence of Methanosphaera stadtmanae Reveals Why This Human Intestinal Archaeon Is Restricted to Methanol and H 2 for Methane Formation and ATP Synthesis. J Bacteriol. 2006;188(2):642-658. doi:10.1128/jb.188.2.642-658.2006
4. McNeil N. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39(2):338-342. doi:10.1093/ajcn/39.2.338
5. Seo M, Heo J, Yoon J et al. Methanobrevibacter attenuation via probiotic intervention reduces flatulence in adult human: A non-randomised paired-design clinical trial of efficacy. PLoS One. 2017;12(9):e0184547. doi:10.1371/journal.pone.0184547
6. Baijal R, Tandon R. Effect of lactase on symptoms and hydrogen breath levels in lactose intolerance: A crossover placebo‐controlled study. JGH Open. 2020;5(1):143-148. doi:10.1002/jgh3.12463
7. García-Collinot G, Madrigal-Santillán E, Martínez-Bencomo M et al. Effectiveness of Saccharomyces boulardii and Metronidazole for Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Dig Dis Sci. 2019;65(4):1134-1143. doi:10.1007/s10620-019-05830-0
8. Kim G, Deepinder F, Morales W et al. Methanobrevibacter smithii Is the Predominant Methanogen in Patients with Constipation-Predominant IBS and Methane on Breath. Dig Dis Sci. 2012;57(12):3213-3218. doi:10.1007/s10620-012-2197-1
9. Jangi S, Gandhi R, Cox L et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1). doi:10.1038/ncomms12015
10. Nkamga V, Henrissat B, Drancourt M. Archaea: Essential inhabitants of the human digestive microbiota. Hum Microb J. 2017;3:1-8. doi:10.1016/j.humic.2016.11.005
11. Pérez-Cobas A, Ginevra C, Rusniok C, Jarraud S, Buchrieser C. Legionella pneumophilainfection and antibiotic treatment engenders a highly disturbed pulmonary microbiome with decreased microbial diversity. 2019. doi:10.1101/808238
12. Sogodogo E, Fellag M, Loukil A et al. Nine Cases of Methanogenic Archaea in Refractory Sinusitis, an Emerging Clinical Entity. Front Public Health. 2019;7. doi:10.3389/fpubh.2019.00038
13. Grine G, Lotte R, Chirio D et al. Co-culture of Methanobrevibacter smithii with enterobacteria during urinary infection. EBioMedicine. 2019;43:333-337. doi:10.1016/j.ebiom.2019.04.037
14. Belay N, Mukhopadhyay B, Conway de Macario E, Galask R, Daniels L. Methanogenic bacteria in human vaginal samples. J Clin Microbiol. 1990;28(7):1666-1668. doi:10.1128/jcm.28.7.1666-1668.1990
15. Grine G, Drouet H, Fenollar F, Bretelle F, Raoult D, Drancourt M. Detection of Methanobrevibacter smithii in vaginal samples collected from women diagnosed with bacterial vaginosis. European Journal of Clinical Microbiology & Infectious Diseases. 2019;38(9):1643-1649. doi:10.1007/s10096-019-03592-1
16. Djemai K, Gouriet F, Michel J, Radulesco T, Drancourt M, Grine G. Methanobrevibacter smithii tonsillar phlegmon: a case report. New Microbes New Infect. 2021;42:100891. doi:10.1016/j.nmni.2021.100891
17. Pascal V, Pozuelo M, Borruel N et al. A microbial signature for Crohn's disease.
18. Knobbe T, Douwes R, Kremer D et al. Altered Gut Microbial Fermentation and Colonization with Methanobrevibacter smithii in Renal Transplant Recipients. J Clin Med. 2020;9(2):518. doi:10.3390/jcm9020518
19. Ramezani A, Nolin T, Barrows I et al. Gut Colonization with Methanogenic Archaea Lowers Plasma Trimethylamine N-oxide Concentrations in Apolipoprotein e−/− Mice. Sci Rep. 2018;8(1). doi:10.1038/s41598-018-33018-5
20. Million M, Maraninchi M, Henry M et al. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes. 2011;36(6):817-825. doi:10.1038/ijo.2011.153
21. Samuel B, Gordon J. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proceedings of the National Academy of Sciences. 2006;103(26):10011-10016. doi:10.1073/pnas.0602187103
22. Mbakwa C, Penders J, Savelkoul P et al. Gut colonization with methanobrevibacter smithii is associated with childhood weight development. Obesity. 2015;23(12):2508-2516. doi:10.1002/oby.21266
23. Visconti A, Le Roy C, Rosa F et al. Interplay between the human gut microbiome and host metabolism. Nat Commun. 2019;10(1). doi:10.1038/s41467-019-12476-z
24. Ruaud A, Esquivel-Elizondo S, de la Cuesta-Zuluaga J et al. Syntrophy via Interspecies H 2 Transfer between Christensenella and Methanobrevibacter Underlies Their Global Cooccurrence in the Human Gut. mBio. 2020;11(1). doi:10.1128/mbio.03235-19
25. Camara A, Konate S, Tidjani Alou M et al. Clinical evidence of the role of Methanobrevibacter smithii in severe acute malnutrition. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-84641-8
26. Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS One. 2009;4(9):e7125. doi:10.1371/journal.pone.0007125
27. Ghoshal U, Shukla R, Srivastava D, Ghoshal U. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver. 2016;10(6):932-938. doi:10.5009/gnl15588
Categories: digestive health Features Research Tags: health research weight-loss wellness